What Tooth Enamel Is Made Up Of: Composition, Chemical Formula & Its pH
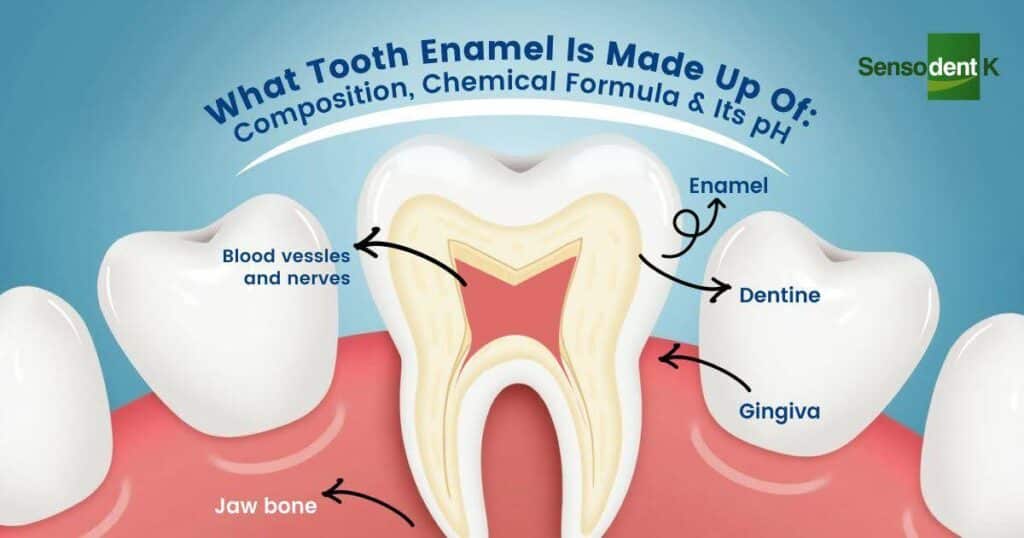
What Tooth Enamel Is Made Up Of: Composition, Chemical Formula & Its pH Tooth enamel is the hardest and most mineralized substance in the human body, designed to protect our teeth from daily wear, chewing forces, and exposure to acids. But what is tooth enamel made of exactly? Understanding its structure helps us appreciate why it’s so resilient yet still vulnerable to erosion and decay. This guide dives into the composition of tooth enamel, how it is built, and why maintaining its strength is crucial for lifelong oral health. From a scientific perspective, tooth enamel is made up of densely packed minerals, primarily hydroxyapatite, which gives it its hardness and durability. The tooth enamel chemical formula is Ca₁₀(PO₄)₆(OH)₂, and its natural pH of tooth enamel plays a key role in determining when demineralization or decay begins. By understanding these details, we can better protect our teeth and adopt habits that support strong, healthy enamel for years to come. Related Blog: What is Tooth Enamel: Protection, Restoration & Care – A Complete Guide What Is The Primary Chemical Composition Of Tooth Enamel? When we think about strong, healthy teeth, the first thing that comes to mind is tooth enamel. But what is tooth enamel made up of? At its core, enamel is mostly inorganic, meaning it is built from minerals rather than living cells. This mineral-rich structure makes it the hardest substance in the human body, even tougher than bone. Understanding the chemical composition of tooth enamel helps us see why it protects teeth so effectively—yet also why it can be vulnerable to acid attacks and erosion. Scientists explain that tooth enamel is made up of several key components, each serving a unique role in protecting our teeth. Here’s a breakdown of the primary elements: Hydroxyapatite (Calcium Phosphate Crystals) The bulk of tooth enamel—about 95–97%—is made up of hydroxyapatite, a crystalline calcium phosphate. This gives enamel its strength and resilience. The tooth enamel chemical formula is Ca₁₀(PO₄)₆(OH)₂, which shows how calcium, phosphate, and hydroxide ions come together to form this highly durable structure. These tightly packed crystals act as a shield against everyday wear from chewing and biting. Water (2–3%) While enamel is mostly mineral, it still contains a small percentage of water. This helps in the diffusion of ions in and out of enamel, especially during processes like demineralization and remineralization. The presence of water is also why enamel can slowly erode when exposed to acidic foods and drinks. Organic Proteins (1–2%) Enamel has a minimal amount of organic material compared to other parts of the tooth, but the proteins present play a critical role in guiding enamel formation during tooth development. Though small in quantity, they help in the structural organization of hydroxyapatite crystals. In short, tooth enamel is made of hydroxyapatite crystals with small amounts of water and proteins. This unique balance makes it incredibly strong, but its mineral-based nature also explains why enamel cannot regenerate once it’s lost. What Is The Chemical Formula Of Hydroxyapatite In Tooth Enamel? The strength of tooth enamel comes mainly from hydroxyapatite, a mineral that makes up the bulk of its structure. If you’ve ever wondered what tooth enamel is made up of on a microscopic level, the answer lies in these tiny, tightly packed crystals. Their arrangement is what gives enamel its exceptional hardness, allowing it to protect teeth from the constant pressure of chewing and the daily wear and tear. The tooth enamel chemical formula for hydroxyapatite is Ca₁₀(PO₄)₆(OH)₂. This means each unit of hydroxyapatite is formed from ten calcium ions, six phosphate groups, and two hydroxide ions. Together, they create a dense, crystalline lattice that resists physical damage. However, this same mineral structure is sensitive to acids. When exposed to acidic foods or bacteria, the hydroxide and phosphate ions can dissolve, weakening the enamel over time. Tooth enamel is made of hydroxyapatite crystals arranged in a way that balances incredible strength with vulnerability to acid erosion. This is why maintaining the right balance of minerals through diet, fluoride, and oral care is so crucial to protecting your enamel for life. How Does The Enamel Chemical Formula Vary In Real Life? While textbooks describe enamel as being made of pure hydroxyapatite with the chemical formula Ca₁₀(PO₄)₆(OH)₂, in reality, things aren’t so simple. In the human mouth, enamel doesn’t exist in a perfectly uniform state—it is influenced by the minerals we consume, our diet, saliva composition, and even the natural aging process. This means the actual chemical composition of tooth enamel can vary slightly from person to person. For example, elements like fluoride, magnesium, carbonate, and sodium often substitute parts of the standard hydroxyapatite structure. When fluoride ions replace hydroxide (OH⁻) ions, they create a stronger version called fluoroapatite, which is more resistant to acid attack. On the other hand, higher levels of carbonate substitution can make enamel weaker and more prone to erosion. This explains why lifestyle factors and local water quality can impact how strong your enamel is. So while tooth enamel is made up of hydroxyapatite at its core, its real-life chemical structure is constantly adapting to your environment and habits. This dynamic nature is what makes proper oral care, diet, and mineral balance so essential to maintaining long-lasting enamel strength. What Role Do Water And Organic Matter Play In Enamel Composition? Although enamel is the hardest tissue in the human body, it is not completely mineralized. In fact, about 3–4% of enamel is made up of water and organic matter. While this may seem like a small fraction compared to the overwhelming presence of hydroxyapatite, these components play an essential role in enamel’s structure and function. The water content in enamel helps with ion transport, allowing calcium and phosphate ions to move within the enamel structure. This is vital for natural remineralization and repair, especially when the enamel is exposed to acidic challenges from food and drinks. Even though enamel does not regenerate like other tissues, water helps maintain its dynamic balance. Meanwhile, the organic matter—primarily